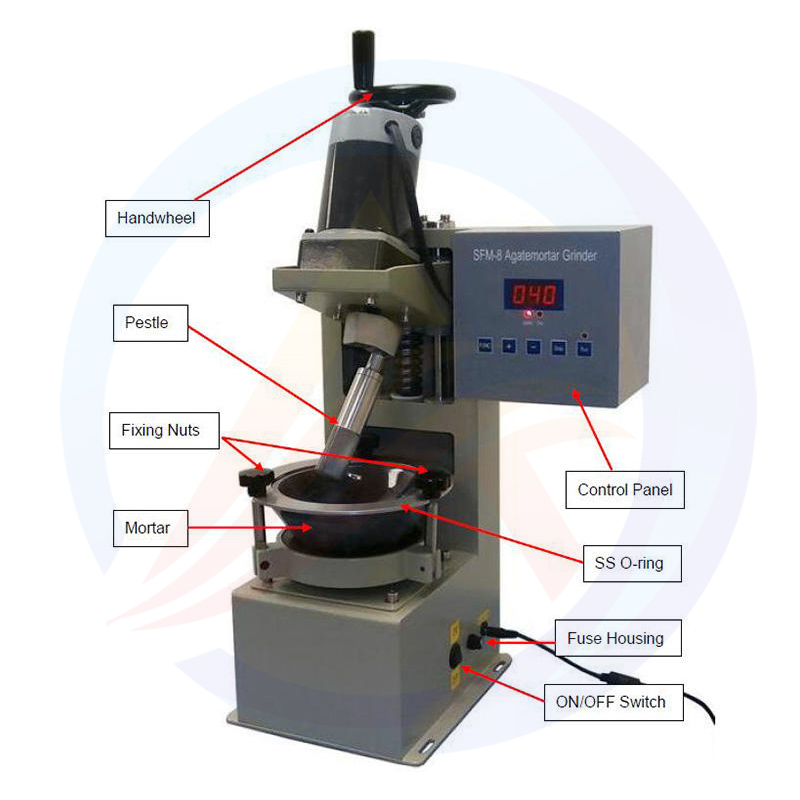
Lithium cobalt oxide has emerged as a pivotal cathode material in lithium-ion battery technology, playing an indispensable role in modern energy storage systems. With the chemical formula LiCoO₂, a molecular weight of 97.87, and CAS registry number 12190-79-3, this black, odorless powder exhibits remarkable thermal stability and electrochemical performance, making it particularly suitable for applications in consumer electronics, electric vehicles, and grid-scale energy storage solutions. The material's high energy density and stable charge-discharge characteristics have cemented its position in the battery industry, though its potential health and environmental hazards necessitate rigorous safety protocols throughout its lifecycle.
The primary composition of LiCoO₂ consists of lithium cobalt oxide with a purity exceeding 95%. While chemically stable under normal conditions, the fine particulate nature of the material presents specific handling challenges, including dust explosion hazards and potential health risks from prolonged exposure. Occupational safety studies indicate that LiCoO₂ may cause allergic skin reactions and respiratory sensitization, with symptoms ranging from localized irritation to more systemic effects. Skin contact can result in erythema, blistering, and pruritus, while ocular exposure may lead to conjunctival irritation, corneal abrasion, and lacrimation. Inhalation of particulate matter represents a significant exposure pathway, potentially causing dyspnea, wheezing, and other respiratory distress symptoms. Of particular concern is the material's classification as containing potentially carcinogenic components, warranting stringent exposure controls in industrial settings.
Engineering controls and personal protective equipment form the foundation of safe Lithium cobalt oxide handling practices. Effective local exhaust ventilation systems must be implemented in processing areas to maintain airborne concentrations below the threshold limit value of 0.02 mg/m³ (as cobalt) established by ACGIH. Personnel handling the material require comprehensive personal protective equipment, including NIOSH-approved respirators with organic vapor cartridges, chemical-resistant gloves meeting EN374 standards, and full-body impervious clothing. Eye protection must conform to ANSI Z87.1 requirements, with sealed goggles recommended for operations generating airborne particulates. Storage protocols dictate maintenance of dry, well-ventilated environments with temperature controls to prevent container pressurization, while transportation procedures emphasize secondary containment measures despite the material's non-hazardous classification under current transport regulations.
Emergency response procedures for lithium cobalt oxide exposure scenarios follow established hazardous materials protocols. Dermal contamination requires immediate removal of contaminated garments followed by copious irrigation with tepid water for at least 15 minutes, with particular attention to preventing material transfer to mucous membranes. Ocular exposure necessitates continuous flushing using emergency eyewash stations, with eyelid retraction to ensure complete decontamination. Inhalation incidents mandate immediate removal to fresh air and supplemental oxygen administration if respiratory distress develops. Gastrointestinal exposure management focuses on oral decontamination without emesis, as aspiration risks outweigh potential benefits of gastric emptying. Medical surveillance programs should monitor for delayed hypersensitivity reactions and potential cobalt accumulation in exposed workers.
Environmental considerations surrounding lithium cobalt oxide remain an area of ongoing research, with current data gaps regarding ecotoxicity profiles and long-term environmental fate. Preliminary studies suggest the material exhibits low solubility in aqueous systems, though its persistence in various environmental compartments requires further investigation. Regulatory frameworks governing lithium cobalt oxide disposal vary by jurisdiction but universally prohibit release into municipal wastewater systems or natural water bodies. Best practices advocate for specialized waste treatment facilities capable of metal recovery, aligning with circular economy principles for critical battery materials.
The regulatory landscape for lithium cobalt oxide continues to evolve in response to advancing toxicological understanding and environmental concerns. Current compliance requirements span multiple legislative domains, including occupational health and safety regulations, chemical control statutes, and waste management directives. Manufacturers and end-users must maintain vigilance regarding evolving classification systems, particularly as global harmonization of hazard communication standards progresses. The European Union's REACH regulation and similar frameworks in other regions increasingly emphasize the need for comprehensive risk assessments throughout the material's lifecycle.
Future research directions should prioritize the development of advanced characterization techniques to better understand exposure biomarkers and long-term health effects. Parallel efforts in material science aim to develop cobalt-reduced or cobalt-free alternatives that maintain performance characteristics while mitigating health and environmental concerns. Life cycle assessment methodologies will prove critical in evaluating the sustainability trade-offs between conventional lithium cobalt oxideand emerging cathode chemistries.
In conclusion, while lithium cobalt oxide remains a cornerstone of contemporary energy storage technology, its safe utilization demands a multidisciplinary approach integrating materials science, occupational health, and environmental stewardship. Continued advances in exposure monitoring technologies, coupled with rigorous adherence to safety protocols, can effectively mitigate risks while enabling the material's ongoing contribution to global electrification efforts. The transition toward sustainable energy systems will require balanced consideration of LiCoO₂'s technical merits against its hazard profile, with research and innovation playing pivotal roles in optimizing this critical balance.