I. Overview of Sodium-Ion Batteries
Sodium-ion batteries are a type of battery that completes charging and discharging by relying on the movement of sodium ions between the positive and negative electrodes, with a working principle similar to that of lithium-ion batteries. A sodium-ion battery is mainly composed of a positive electrode, a negative electrode, an electrolyte, a separator, and a current collector.During charging, Na⁺ is extracted from the positive electrode, passes through the separator, and embeds into the negative electrode to combine with electrons. During discharging, Na⁺ is extracted from the negative electrode, passes through the separator, and embeds into the positive electrode, while electrons are transferred from the negative electrode to the positive electrode through an external circuit. Finally, a redox reaction occurs in the positive electrode to restore the sodium-rich state.
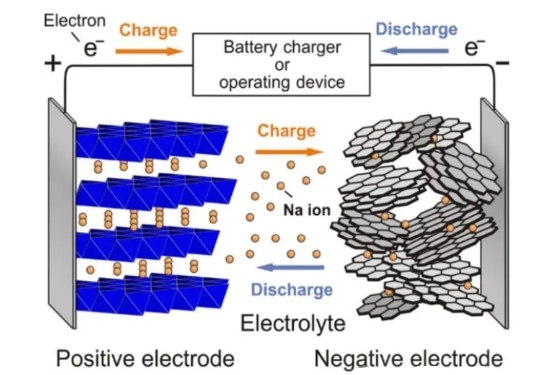
Schematic diagram of charge and discharge of sodium-ion batteries
II. Three Technical Routes
Compared with lithium-ion batteries, the most significant change in sodium-ion batteries lies in the cathode materials, whose performance is also a key factor determining the battery's energy density, safety, and cycle life. Sodium ions have a larger mass and radius than lithium ions, resulting in lower ionic diffusion rates. This is reflected in slightly inferior theoretical capacity and reaction kinetics in battery performance, which require breakthroughs in cathode materials to address these issues. At present, the technical route for cathode materials has not yet been determined, but layered oxides, Prussian blue analogs, and polyanion compounds are three promising routes expected to stand out.
III. Layered Oxides
The general formula for layered oxides is NaxMO2, where M refers to transition metal elements, such as vanadium (V), chromium (Cr), manganese (Mn), iron (Fe), cobalt (Co), nickel (Ni), copper (Cu), etc. Among them, manganese (Mn) and iron (Fe), which are abundant in resources, are the most common. Transition metal oxides can be further classified into two types: layered and tunnel. When the sodium content is low (x<0.5), the tunnel structure is mainly present. When the sodium content is relatively high, it is generally dominated by a layered structure, with Na+ located between the layers, forming a layered structure in which MO2 layers and sodium layers are alternately arranged.
Iv. Prussian Blue analogues
The general formula for Prussian blue analogues is NaxMA[MB(CN)6]·zH2O. MA and MB represent transition metal elements, mainly iron (Fe), cobalt (Co), nickel (Ni), manganese (Mn), etc. Due to the unique open framework and three-dimensional macroporous structure of Prussian blue compounds, they are suitable for the migration and storage of sodium ions. In terms of advantages, iron-based Prussian blue and manganese-based Prussian blue have the advantages of abundant raw materials, low cost, high specific capacity, high rate performance and excellent electrochemical stability. In terms of disadvantages, as the current production methods mostly adopt the co-precipitation method, many crystalline water and Fe(CN)6 structural defects are often produced. The crystalline water is prone to occupy the sodium storage sites and sodium ion deintercalation channels in the crystal, resulting in a decrease in the sodium ion content in the material and a reduction in the sodium ion migration rate. Structural defects and crystalline water of Fe(CN)6 can cause structural collapse during the charging and discharging process of the material, affecting the cycling performance of the material.
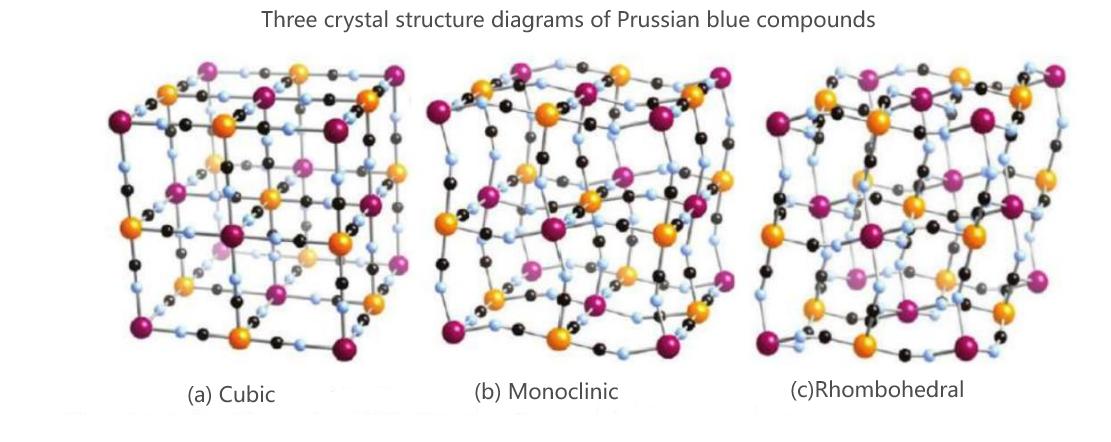
The production processes of Prussian blue compounds mainly include co-precipitation and hydrothermal synthesis. Among them, co-precipitation is the most common method, which has the advantages of simple preparation process, no need for high-temperature treatment and easy acquisition of pure phase products. However, at present, the co-precipitation method still has two problems. One is the long preparation time; The second is the low output. The hydrothermal synthesis method shares many similarities with the co-precipitation method. It has the advantages of short reaction time and uniform distribution of material particles. However, at present, the hydrothermal synthesis method has three disadvantages. First, the reaction process takes place in a closed system, and the reaction process cannot be directly observed. Second, there are high-temperature and high-pressure steps, which have high requirements for production equipment. Thirdly, the process is cumbersome and not suitable for industrial production.
V. Polyanionic compounds
The general formula of polyanionic compounds is NaxMy[(XOm)n-]z, where M is a metal ion with a variable valence state and X is elements such as P, S and V. It has the advantages of good stability, cycling performance and safety, but there are problems of low specific capacity and poor conductivity. According to their different structures, they can be classified into olivine-structured phosphates, NASCICON (Na+ fast ion conductor) compounds and phosphate compounds.
The preparation method of olivine-structured NaFePO4 as the cathode material for sodium-ion batteries is similar to that of lithium iron phosphate. Its theoretical capacity is 154mAh/g and the working voltage is 2.9V. However, its own electrical conductivity is relatively low and it only has one-dimensional Na+ diffusion channels, which affects its actual performance. Currently, the electrical conductivity is improved through carbon coating or ion substitution. Nascicon-structured compounds are fast ionic conductors with a theoretical specific capacity of approximately 120mAh/g and an operating voltage of about 3.3V. They feature a 3D framework structure, a high ion diffusion rate, and good kinetic and cycling stability. However, when the pentavalent V is introduced, it is often toxic and poses a great threat to human health, which to some extent limits its large-scale use.
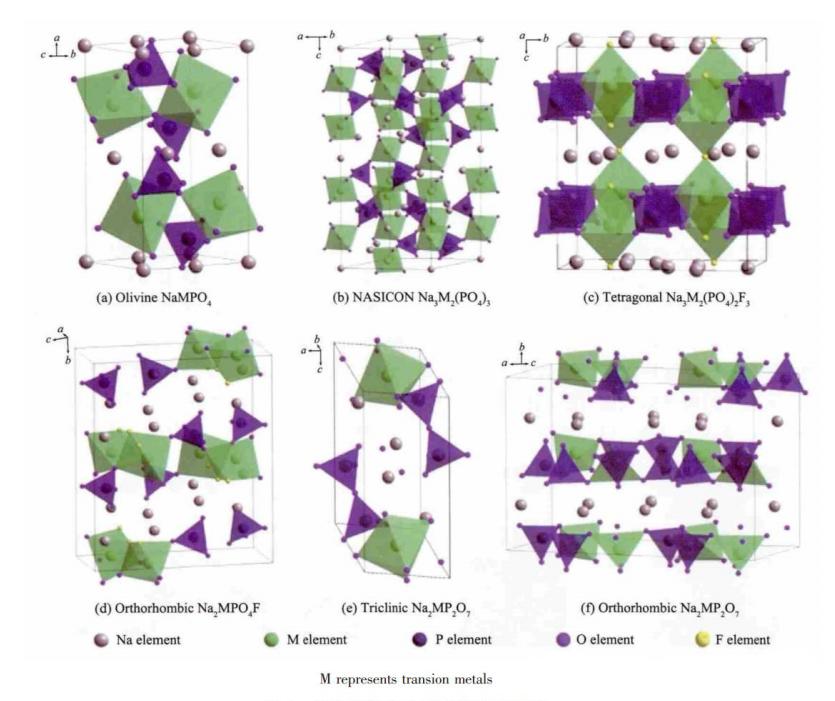
Crystal structures of various polyanionic cathode materials